de broglie wave equation derivation|De Broglie Wavelength: Definition, Equation & How to Calculate : Tuguegarao Calculate the de Broglie wavelength of an electron of kinetic energy 500 eV. Solution: The kinetic energy of an electron 500 eV means the electron is accelerated by the potential . On the other hand, Circle network limited has reached the dept of heart by providing their free FTP server site as well. So, if you want more ftp server like as circle FTP server then check our website homepage. Circle Network FTP. 15.1.1.1. 1.1.1.5. Circle FTP | 15.1.1.1. This IP address 15.1.1.1 is directly connected to circle FTP, so if a .
PH0 · de Broglie equation, derivation, and its Significance
PH1 · de Broglie Wavelength: Definition, Hypothesis, and Equation
PH2 · de Broglie Wavelength
PH3 · de Broglie Equation
PH4 · Quantum Physics I, Lecture Note 4
PH5 · Deriving the de Broglie Wavelength
PH6 · De Broglie Wavelength: Definition, Equation & How to Calculate
PH7 · De Broglie Wavelength : Derivation, Formula and Solved Examples
PH8 · 6.6: De Broglie’s Matter Waves
PH9 · 5.8: de Broglie Wave Equation
PH10 · 13.3: Free Particles and the de Broglie Wavelength
Dual-channel memory offers a significant boost in memory bandwidth, but why is that important? If you're curious about memory channels, especially because you want to build or upgrade a PC, we’ll explain all the most
de broglie wave equation derivation*******By using a series of substitution de Broglie hypothesizes particles to hold properties of waves. Within a few years, de Broglie's hypothesis was tested by scientists shooting electrons and rays of lights through slits.In 1924, French scientist Louis de Broglie (1892-1987) derived an equation that .de broglie wave equation derivation De Broglie Wavelength: Definition, Equation & How to CalculateToday, this idea is known as de Broglie’s hypothesis of matter waves. In 1926, De .De Broglie Wavelength: Definition, Equation & How to CalculateDerivation. de Broglie derived the above relationship as follows: 1) E = hν for a photon and λν = c for an electromagnetic wave. 2) E = mc 2, means λ = h/mc, which is equivalent to .Calculate the de Broglie wavelength of an electron of kinetic energy 500 eV. Solution: The kinetic energy of an electron 500 eV means the electron is accelerated by the potential . In 1924, French scientist Louis de Broglie (1892-1987) derived an equation that described the wave nature of any particle. Particularly, the wavelength \(\left( . Today, this idea is known as de Broglie’s hypothesis of matter waves. In 1926, De Broglie’s hypothesis, together with Bohr’s early quantum theory, led to the . Table of Contents. de Broglie Equation Definition. de Broglie wave equation derivation. de Broglie equation significance. de Broglie Hypothesis video. . de Broglie Equation. A fundamental equation core to de Broglie hypothesis establishes the relationship between a particle’s wavelength and momentum. This .
1 de Broglie wavelength and Galilean transformations We have seen that to any free particle with momentum p, we can associate a plane wave, or a \matter wave", with de .
The de Broglie wavelength indicates that wavelength is inversely proportional to momentum. For a non-relativistic particle (which is implied here, as the Schr¨odinger . Derivation of the de Broglie phase wave through the standing (Doppler shifted) electromagnetic waves inside the particles is described in the article [6]. In addition, in the article [ 7 ] is assumed that inside a particle there is a rotary electromagnetic wave.
In this case, λ gives the wave length of the electron (or particle) of momentum p and is known as the de Broglie wavelength. Thus, substituting the values of Planck’s constant h and momentum of the particle p in equation (1), we get de Broglie wavelength of the wave associated with the moving particle. Quantum mechanics takes de Broglie’s idea of matter waves to be the fundamental property of all particles and gives it a statistical interpretation. According to this interpretation, a wave that is associated with a particle carries information about the probable positions of the particle and about its other properties.
Thus, the derivation of De Broglie Equation questions the matter’s wave properties, mainly electrons. λ = h/mv. In this equation, λ = wavelength, h = Planck’s constant. m = particle’s mass. v = velocity of the particle. Derivation of De Broglie Equation. For the derivation of De Broglie’s equation, we need to follow the two equations .Compton’s formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie’s hypothesis of matter waves.In 1926, De Broglie’s .
The wavelength of the matter waves is referred to as the de Broglie wavelength. Through Planck's constant, this equation connects the wave character ( wavelength) and the particle character (momentum p). Derivation of De Broglie Wavelength Formula or De Broglie Equation. We know from Einstein's mass-energy .
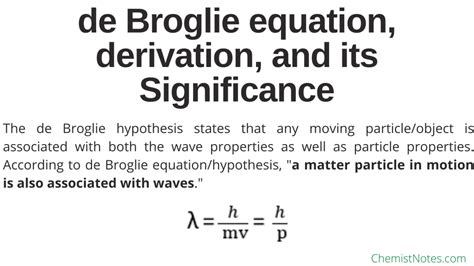
Through de Broglie’s relationship, we now had a wave theory of matter. The ‘Lambda (λ)’ here represents the wavelength of the particle and ‘p’ represents the momentum of the particle.
The effect of a potential on a de Broglie wave was considered by Sommerfeld in an attempt to generalize the rather restrictive conditions in Bohr’s model of the atom. . Adding waves in this way leads to a more general derivation of the formula \(d\omega/dk\) for the group velocity. The standard approach is to replace the sum over .In general, the longer the wavelength of a wave (i.e. the larger \(\lambda\) is), the easier it is to see interference effects. The de Broglie wavelength indicates that wavelength is inversely proportional to momentum. For a non-relativistic particle (which is implied here, as the Schr¨odinger equation assumes non-relativistic particles), \(p .

Understanding De Broglie Equation Derivation In this article we are going to learn about Understanding De Broglie Equation Derivation, de broglie wavelength formula and derivation, wave-particle duality, momentum, de Broglie Hypothesis, De Brogile Wavelength, Significance of De Broglie Equation and more.
Key learnings: Schrödinger Wave Equation Definition: The Schrödinger Wave Equation is a fundamental formula in quantum mechanics that describes how the quantum state of a physical system . De Broglie’s Hypothesis and Equation. Louis-de-Broglie explained the concept of de-Broglie waves in the year 1923. In his thesis, he suggested that any moving particle, whether microscopic or macroscopic, will be related to a wave character. This was later experimented with and proved by Davisson and Germer within the year 1927.
De Broglie’s proposal of a wave nature for all particles initiated a remarkably productive era in which the foundations for quantum mechanics were laid. . published four papers in which the wave nature of particles was treated explicitly with wave equations. At the same time, many others began important work. Among them was .
3 Wave/particle duality and de Broglie waves; 4 Particles at boundaries, potential steps, barriers, and in quantum wells; 5 The harmonic oscillator and photons; 6 The hydrogen atom; 7 Multi-electron ions and the periodic table; 8 Interaction of atoms with electromagnetic radiation; 9 Simple molecular orbitals and crystalline structures The answer lies in the numerator of de Broglie’s equation, which is an extremely small number. As you will calculate in Example 4, Planck’s constant . the so-called wave–particle duality. Louis de Broglie showed that the wavelength of a particle is equal to Planck’s constant divided by the mass times the velocity of the particle. A fundamental equation core to de Broglie hypothesis establishes the relationship between a particle’s wavelength and momentum.This equation is the cornerstone of quantum mechanics and sheds light on the wave-particle duality of matter. It revolutionizes our understanding of the behavior of particles at the quantum level. Using simple algebra and some of Einstein’s equations (for mass-energy and the photoelectric effect), I derive De Broglie’s equation for the wavelength of hi.
The above equation is known as de Broglie relationship and the wavelength, λ is known as de Broglie wavelength. Diffraction of electron beams explains the de Broglie relationship as diffraction is the property of waves. An electron microscope is a common instrument illustrating this fact. Thus, every object in motion has a wavelike character.
AZ’s offline options are very restricted for eighteen-year-olds due to their minimum gambling age being 21. This means residents must wait until they are at least 21 to participate in gambling at tribal casinos, playing the state’s lottery, and betting on horse and dog races. We created a page just to explain gambling laws for 18+ casinos.
de broglie wave equation derivation|De Broglie Wavelength: Definition, Equation & How to Calculate